Abstract
Introduction
Liposomal doxorubicin and romidepsin have single agent activity in PTCL and CTCL. In pre-clinical studies, we demonstrated that romidepsin had synergistic effects in combination with doxorubicin. The aim of this study is to determine the safety and feasibility of the combination of romidepsin and liposomal doxorubicin in patients with relapsed/refractory CTCL and PTCL.
Materials and Methods:
This is a multi-center, single arm, open-label phase I dose-escalation study. Patients with relapsed/refractory CTCL (stage IIA-IV), or PTCL of any stage or subtype who received at least one systemic therapy are eligible. The primary endpoint is to identify the maximum tolerated dose (MTD) of romidepsin in combination with liposomal doxorubicin and the secondary endpoint is to determine the overall response rate (ORR) of this combination. Patients were treated with liposomal doxorubicin 20 mg/m2 on day 1 and romidepsin at four dose levels on days 1, 8 and 15, every 28 days, until 2 cycles beyond the best response, 8 cycles, disease progression (PD), or intolerability; whichever comes first. Dose levels of romidepsin include 8, 10, 12 and 14 mg/m2. Dose limiting toxicity (DLT) is defined as any adverse event during therapy including: grade 5 toxicity, any grade 4 hematologic toxicity lasting >7 days (except neutropenia), or any grade 4 non-hematologic toxicity according to CTCAE version 4.0. The MTD was defined as the highest tested dose level where two or more participants experienced a DLT utilizing a standard 3+3 design. Response was evaluated using the Cheson criteria in PTCL and patients with nodal disease and the mSWAT was utilized in CTCL. Pre-specified in the protocol, after determination of the MTD of romidepsin, up to 12 patients will be treated in a dose-expansion cohort if efficacy is noted.
Results:
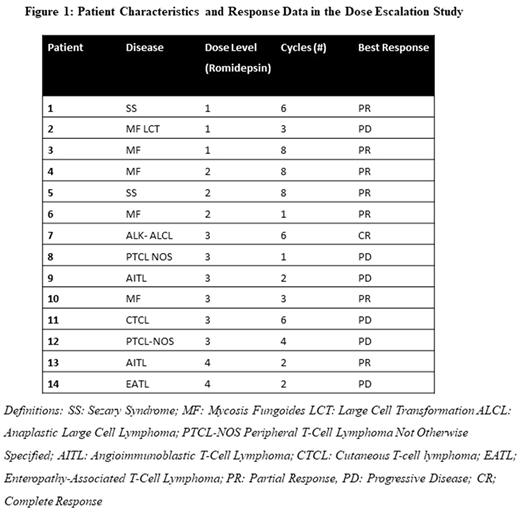
Fourteen patients (5 PTCL, 9 CTCL) were treated at four dose-levels with a median 4 cycles (range 1-8) of therapy. Clinical data regarding these patients is included in Figure 1. Grade 3/4 non-hematologic adverse events occurred in 4 patients including 3 infection, 1 fatigue and 1 atrial fibrillation. Four patients experienced grade 3/4 hematologic toxicities: 1 neutropenia, 2 thrombocytopenia and 3 anemia. The MTD of romidepsin was determined to be 12 mg/m2 and the dose limiting toxicity was prolonged (>7 days) grade 4 thrombocytopenia. The ORR was 57%, including 2 complete responses and 6 partial responses.
Given these results, three additional patients have been treated in a pre-specified dose-expansion cohort. For the 17 total patients (including 3 additional patients treated at the MTD), the ORR was 52%. In CTCL the ORR was 78% (1 CR, 6 PR, 2 PD) and among PTCL patients the ORR was 25% (1 CR, 1 PR, 1 PD).
Conclusion:
Here we report the results of the dose-escalation study of the combination of romidepsin and liposomal doxorubicin with preliminary data on the ORR. This combination has an acceptable toxicity profile at an MTD of 12 mg/m2 for romidepsin and has promising efficacy in both relapsed/refractory PTCL and CTCL. An additional eight patients will be included in a dose-expansion cohort.
Ai: Celgene: Research Funding. Porcu: Kiowa: Research Funding; Miragen: Research Funding; Tetralogic: Research Funding; Celgene: Research Funding; Innate Pharma: Research Funding; Cell Medica: Research Funding; Galderma: Research Funding; Kura: Research Funding. Wieduwilt: Sigma-Tau: Research Funding; Reata Pharmaceuticals: Equity Ownership. Brammer: Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal